Putting the focus on specific rare bleeding disorders
Our approved coagulation factor VIIa (recombinant) is available for the treatment and control of bleeding episodes occurring in adults and adolescents (12 years of age and older) with hemophilia A or B with inhibitors.
What are inhibitors?
Before talking about inhibitors, let's first look at the clotting cascade. The clotting cascade shows the many diferent steps that are needed to produce a clot.
The clotting cascade is activated by the platelet response to the leaky blood vessel. The platelet response is called Primary Hemostasis.

Primary Hemostasis: Platelet response to a bleed
There are two main pathways in the clotting cascade: the intrinsic pathway and the extrinsic pathway. People with hemophilia A are lacking factor VIII, which is a key factor in the intrinsic pathway. To form clots, they need to infuse replacement factor VIII, to supply what the body is missing.
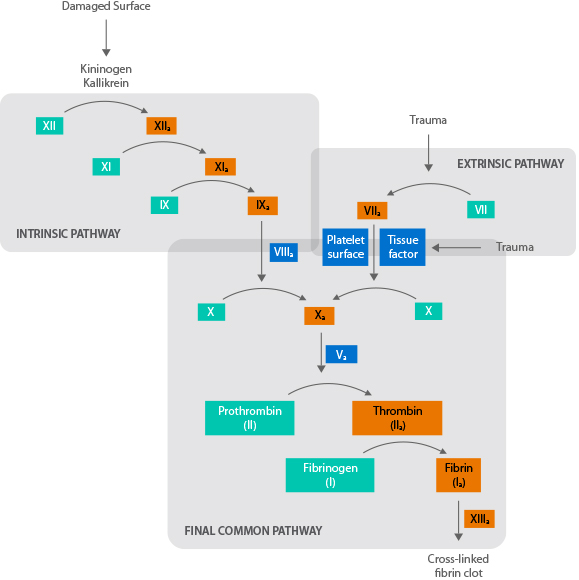
Each step activates the enzyme necessary for the next step.
Sometimes, the body reacts to this replacement factor as an invader and forms an antibody against it. These antibodies are referred to as inhibitors to the infused clotting factor. These inhibitors act to eliminate the foreign body, making the replacement factor ineffective.
Learn More at NHFWho develops an inhibitor?
Approximately 30% of people with severe hemophilia A will develop an inhibitor to infused factor VIII at some point in their lives. Even 5% to 8% of people with mild or moderate hemophilia A develop an inhibitor.
Learn More at NHFHow are bleeds treated in the presence of an inhibitor?
For patients with hemophilia A with low titer inhibitors, therapy with factor VIII can be effective for many bleeding episodes. Higher doses of factor or more frequent infusions may be required to overcome the inhibitor.
When patients have high titer inhibitors, high factor doses become ineffective. At this point, they must switch to an alternate clotting factor(s) to control their bleeding. These factors “bypass” the missing factor VIII to create a functioning clotting system—thus, they are called bypassing agents. FVIIa is normally found in the extrinsic pathway. When given as a treatment, factor VIIa works with platelets at the site of the injury to make clots form to stop bleeding.
How do inhibitors affect the lives of patients?
Inhibitors make life more challenging for someone with hemophilia. New therapies may need to be added, which may cause an adjustment period. Bleeding episodes may take longer to control depending on the severity of the inhibitor. The longer bleeding continues, the higher the chance that blood may find its way into muscles and joints, causing additional damage.
What is HEMA Biologics doing to help?
We recognize that for people living with an inhibitor, every day brings challenges. The primary goal of our approved inhibitor bypassing agent is to advance the treatment and management of hemophilia A with inhibitors to positively affect patients’ lives.
What are inhibitors?
Before talking about inhibitors, let's first look at the clotting cascade. The clotting cascade shows the many diferent steps that are needed to produce a clot.
The clotting cascade is activated by the platelet response to the leaky blood vessel. The platelet response is called Primary Hemostasis.

Primary Hemostasis: Platelet response to a bleed
There are two main pathways in the clotting cascade: the intrinsic pathway and the extrinsic pathway. People with hemophilia B are lacking factor IX, which is a key factor in the intrinsic pathway. To form clots, they need to infuse replacement factor IX, to supply what the body is missing.

Each step activates the enzyme necessary for the next step.
Sometimes, the body reacts to this replacement factor as an invader by forming antibodies to the factor. These antibodies, also known as inhibitors, act to eliminate the foreign protein, making the replacement factor ineffective.
Learn More at NHFWho develops an inhibitor?
Approximately 2% to 3% of people with hemophilia B will develop an inhibitor to infused factor IX. Although they are less common than in hemophilia A, inhibitors can be even more problematic in patients with hemophilia B due to the potential of developing a severe allergic reaction.
Learn More at NHFHow are bleeds treated in the presence of an inhibitor?
For patients with hemophilia B with low titer inhibitors, therapy with factor IX can be effective for many bleeding episodes. Higher doses of factor or more frequent infusions may be required to overcome the inhibitor.
When patients have high titer inhibitors, high factor doses become ineffective. At this point, they must switch to an alternate clotting factor(s) to control their bleeding. These factors “bypass” the missing factor IX to create a functioning clotting system—thus, they are called bypassing agents. FVIIa is normally found in the extrinsic pathway. When given as a treatment, factor VIIa works with platelets at the site of the injury to make clots form to stop bleeding.
How do inhibitors affect the lives of patients?
Inhibitors make life more challenging for someone with hemophilia. New therapies may need to be added, which may cause an adjustment period. Bleeding episodes may take longer to control depending on the severity of the inhibitor. The longer bleeding continues, the higher the chance that blood may find its way into muscles and joints, causing additional damage.
What is HEMA Biologics doing to help?
We recognize that for people living with an inhibitor, every day brings challenges. The primary goal of our approved inhibitor bypassing agent is to advance the treatment and management of hemophilia B with inhibitors to positively affect patients’ lives.
What are inhibitors?
Before talking about inhibitors, let's first look at the clotting cascade. The clotting cascade shows the many diferent steps that are needed to produce a clot.
The clotting cascade is activated by the platelet response to the leaky blood vessel. The platelet response is called Primary Hemostasis.

Primary Hemostasis: Platelet response to a bleed
There are two main pathways in the clotting cascade: the intrinsic pathway and the extrinsic pathway. People with hemophilia A are lacking factor VIII, which is a key factor in the intrinsic pathway. To form clots, they need to infuse replacement factor VIII, to supply what the body is missing.

Each step activates the enzyme necessary for the next step.
Sometimes, the body reacts to this replacement factor as an invader and forms an antibody against it. These antibodies are referred to as inhibitors to the infused clotting factor. These inhibitors act to eliminate the foreign body, making the replacement factor ineffective.
Learn More at NHFWho develops an inhibitor?
Approximately 30% of people with severe hemophilia A will develop an inhibitor to infused factor VIII at some point in their lives. Even 5% to 8% of people with mild or moderate hemophilia A develop an inhibitor.
Learn More at NHFHow are bleeds treated in the presence of an inhibitor?
For patients with hemophilia A with low titer inhibitors, therapy with factor VIII can be effective for many bleeding episodes. Higher doses of factor or more frequent infusions may be required to overcome the inhibitor.
When patients have high titer inhibitors, high factor doses become ineffective. At this point, they must switch to an alternate clotting factor(s) to control their bleeding. These factors “bypass” the missing factor VIII to create a functioning clotting system—thus, they are called bypassing agents. FVIIa is normally found in the extrinsic pathway. When given as a treatment, factor VIIa works with platelets at the site of the injury to make clots form to stop bleeding.
How do inhibitors affect the lives of patients?
Inhibitors make life more challenging for someone with hemophilia. New therapies may need to be added, which may cause an adjustment period. Bleeding episodes may take longer to control depending on the severity of the inhibitor. The longer bleeding continues, the higher the chance that blood may find its way into muscles and joints, causing additional damage.
What is HEMA Biologics doing to help?
We recognize that for people living with an inhibitor, every day brings challenges. The primary goal of our approved inhibitor bypassing agent is to advance the treatment and management of hemophilia A with inhibitors to positively affect patients’ lives.
What are inhibitors?
Before talking about inhibitors, let's first look at the clotting cascade. The clotting cascade shows the many diferent steps that are needed to produce a clot.
The clotting cascade is activated by the platelet response to the leaky blood vessel. The platelet response is called Primary Hemostasis.

Primary Hemostasis: Platelet response to a bleed
There are two main pathways in the clotting cascade: the intrinsic pathway and the extrinsic pathway. People with hemophilia B are lacking factor IX, which is a key factor in the intrinsic pathway. To form clots, they need to infuse replacement factor IX, to supply what the body is missing.

Each step activates the enzyme necessary for the next step.
Sometimes, the body reacts to this replacement factor as an invader by forming antibodies to the factor. These antibodies, also known as inhibitors, act to eliminate the foreign protein, making the replacement factor ineffective.
Learn More at NHFWho develops an inhibitor?
Approximately 2% to 3% of people with hemophilia B will develop an inhibitor to infused factor IX. Although they are less common than in hemophilia A, inhibitors can be even more problematic in patients with hemophilia B due to the potential of developing a severe allergic reaction.
Learn More at NHFHow are bleeds treated in the presence of an inhibitor?
For patients with hemophilia B with low titer inhibitors, therapy with factor IX can be effective for many bleeding episodes. Higher doses of factor or more frequent infusions may be required to overcome the inhibitor.
When patients have high titer inhibitors, high factor doses become ineffective. At this point, they must switch to an alternate clotting factor(s) to control their bleeding. These factors “bypass” the missing factor IX to create a functioning clotting system—thus, they are called bypassing agents. FVIIa is normally found in the extrinsic pathway. When given as a treatment, factor VIIa works with platelets at the site of the injury to make clots form to stop bleeding.
How do inhibitors affect the lives of patients?
Inhibitors make life more challenging for someone with hemophilia. New therapies may need to be added, which may cause an adjustment period. Bleeding episodes may take longer to control depending on the severity of the inhibitor. The longer bleeding continues, the higher the chance that blood may find its way into muscles and joints, causing additional damage.
What is HEMA Biologics doing to help?
We recognize that for people living with an inhibitor, every day brings challenges. The primary goal of our approved inhibitor bypassing agent is to advance the treatment and management of hemophilia B with inhibitors to positively affect patients’ lives.



















