Information for patients and caregivers
HEMA Biologics is focused on building strong bonds with the community suffering with rare bleeding disorders.
Our promise to patients and caregivers
HEMA Biologics is focused on rare bleeding disorders.
We aspire to support patients living with these disorders, partner with the broader community that cares for them, and bring meaningful treatments and services to help better their daily lives.
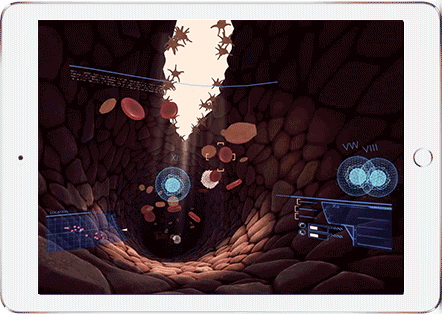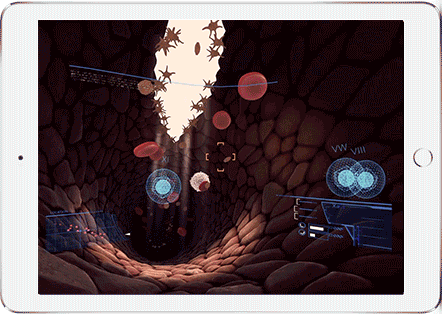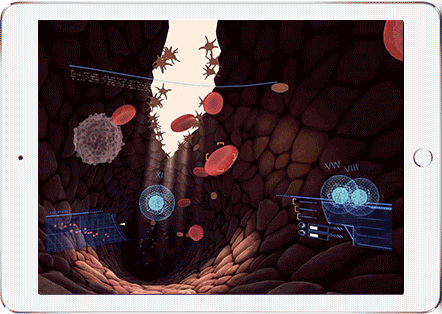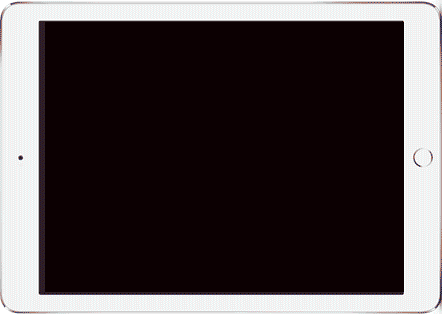
Help Sergeant Factor on his mission to stop a bleed. This fast-paced, informative virtual reality experience puts you behind the controls to stop a bleed. Make sure you get the bleed stopped in time!
Download the app on your phone and enjoy the action anytime!

National Hemophilia Foundation
The National Hemophilia Foundation (NHF) is dedicated to finding better treatments and cures for inheritable bleeding disorders and to preventing the complications of these disorders through education, advocacy, and research.

Hemophilia Federation of America
Hemophilia Federation of America (HFA) member organizations across the country utilize HFA’s collaborative federation to strengthen community support and awareness, develop effective local organizations, and implement valuable community-based programs.

World Federation of Hemophilia
The World Federation of Hemophilia improves and sustains care for people with inherited bleeding disorders around the world.

Comprehensive Health Education Services
Comprehensive Health Education Services (CHES) is dedicated to the education and nurturing of individuals with bleeding disorders, their families, and their medical professionals.

The Coalition for Hemophilia B
The Coalition for Hemophilia B strives to make quality of life the focal point of treatment for people with hemophilia B and their families through education, empowerment, advocacy, and outreach.


















